Help ensure patients are ready to treat a severe low with Gvoke HypoPen®

Gvoke HypoPen Is Well Studied in Patients
Gvoke Was Evaluated in 2 Phase 3 Trials of Adults With T1D1,2
154 subjects received an injection of Gvoke and 157 subjects received an injection of GEK. A total of 152 subjects received both Gvoke and GEK.
In study A, mean plasma glucose at time of administration was 44.8 mg/dL for Gvoke and 45.2 mg/dL for GEK. In Study B, mean plasma glucose at time of administration was 47.7 mg/dL for Gvoke and 48.7 mg/dL for GEK.
The Safety and Efficacy of Gvoke® for Children and Adolescents Was Evaluated in a Phase 3, Open-label Study1,5
Demonstrated Safety and Efficacy
99% (152/154) of adult patients achieved plasma glucose > 70 mg/dL or an increase ≥ 20 mg/dL from baseline in a pooled analysis of Study A and Study B.1
The most common adverse reactions in adults (N=154) were1: nausea (30%), vomiting (16%), injection site edema (7%), and headache (5%).
The mean time to treatment success was 13.8 minutes in the Gvoke group.
Patients experienced initial blood glucose increases in less than 2 minutes3
Post-hoc analysis in adults revealed the median time to initial blood glucose increase was less than 2 minutes.3†
~176 mg/dL mean maximum increase in plasma glucose from an average baseline of 44 mg/dL (insulin-induced severe hypoglycemia), following administration of Gvoke HypoPen.1
† Estimations yielding times earlier than the initial post dose (PD) collection at 5 minutes assume an immediate increase from baseline and cannot account for a potentially delayed onset from time of injection to initial PD response.
100% (30/30) of pediatric patients achieved a target glucose increase of ≥25 mg/dL.1,5
The most common adverse reactions in pediatric patients (N=31) were1: nausea (45%), hypoglycemia (39%), vomiting (19%), headache (7%), hyperglycemia (7%), abdominal pain (3%), injection site discomfort (3%), injection site reaction (3%), and urticaria (3%).
Adverse reactions occur within 12 hours.
Patients experienced initial blood glucose increases in less than 2 minutes3
Patients experienced initial blood glucose increases in less than 2 minutes with Gvoke HypoPen, and no overcorrection.2
~176 mg/dL mean maximum increase in plasma glucose from an average baseline of 44 mg/dL (insulin-induced severe hypoglycemia), following administration of Gvoke HypoPen.
In a human-factors study with simulated emergency conditions, 99% of people were able to give Gvoke HypoPen correctly4
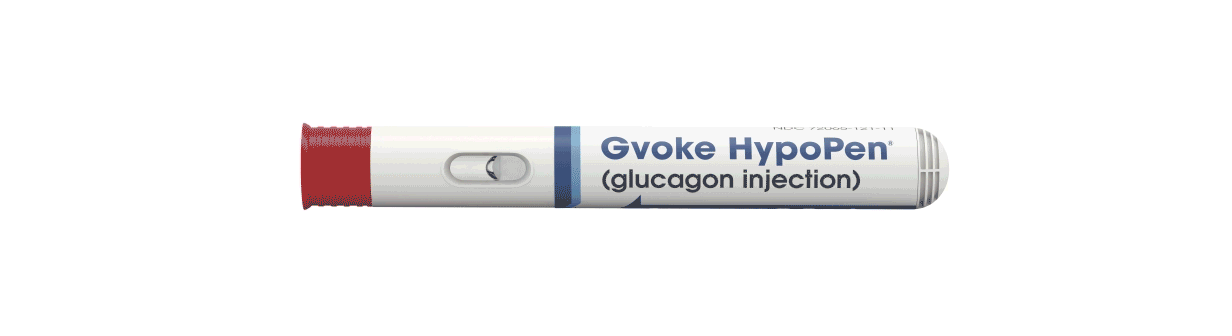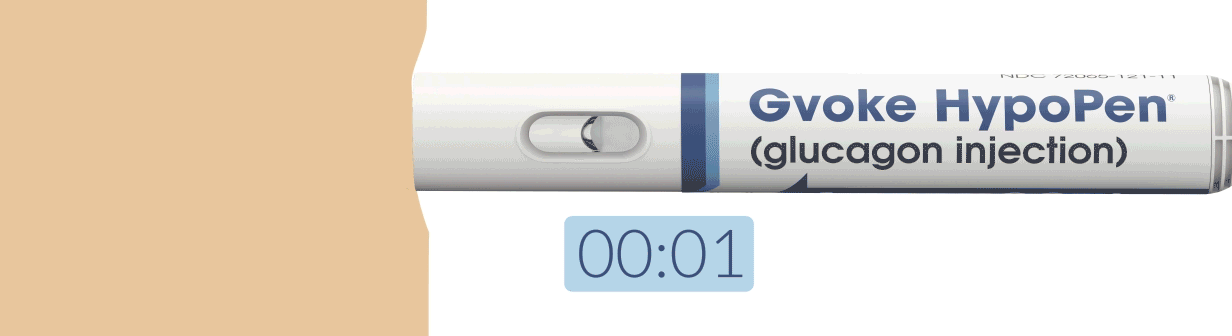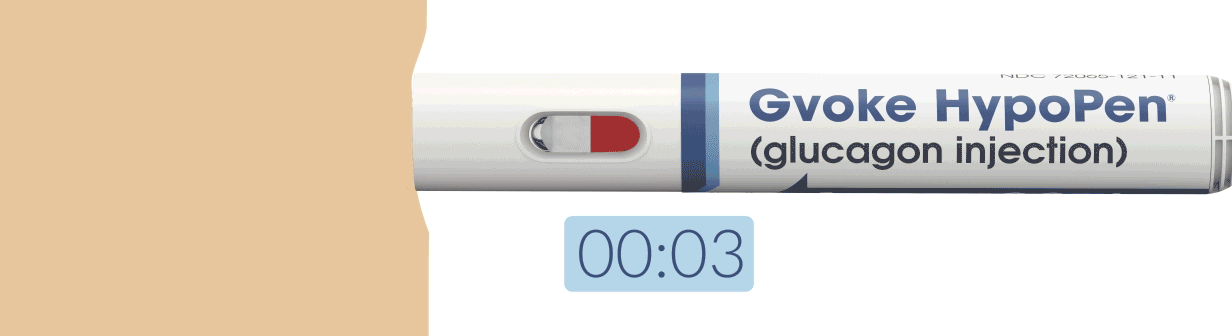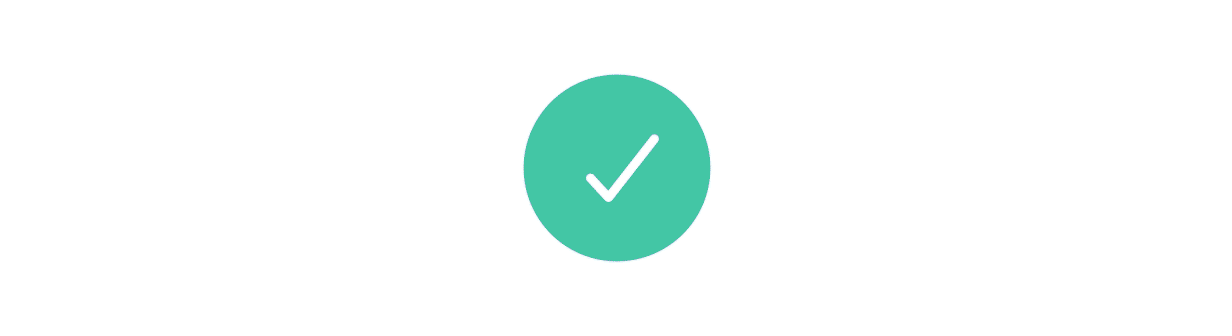
How To Use Gvoke HypoPen in 2 Simple Steps6

Understanding Dosages
Gvoke HypoPen comes in two premeasured dosing options.1
1 mg per 0.2 mL
- For patients ages 12 and older, or children under 12 years old who weigh ≥ 100 lbs
- Lasts 30 months from date of manufacture when stored at room temperature3
0.5 mg per 0.1 mL
- For patients between the ages 2 and 11 who weigh less than 100 lbs
- Lasts 24 months from date of manufacture when stored at room temperature3
Help Patients Save Money on Gvoke HypoPen

Access Financial Support

* In a pooled analysis of 2 clinical studies in adults, mean time to treatment success was 13.8 minutes with treatment success defined as plasma glucose increase from mean value (< 50 mg/dL) at time of glucagon administration to absolute value greater than 70 mg/dL or relative increase of 20 mg/dL or greater.
† Offer not valid for prescriptions reimbursed under Medicaid, a Medicare drug benefit plan, TRICARE, or other federal or state health programs (such as medical assistance programs).
REFERENCES:
- Gvoke [prescribing information]. Chicago, IL: Xeris Pharmaceuticals, Inc.
- Christiansen MP, Cummins M, Prestrelski S, Close NC, Nguyen A, Junaidi K. Comparison of a ready-to-use liquid glucagon injection administered by autoinjector to glucagon emergency kit for the symptomatic relief of severe hypoglycemia: two randomized crossover non-inferiority studies. BMJ Open Diabetes Res Care. 2021;9(1):e002137. doi:10.1136/bmjdrc-2021-002137
- Data on file. Xeris Pharmaceuticals, Inc
- Valentine V, Newswanger B, Prestrelski S, Andre AD, Garibaldi M. Human Factors Usability and Validation Studies of a Glucagon Autoinjector in a Simulated Severe Hypoglycemia Rescue Situation. Diabetes Technol Ther. 2019;21(9):522-530. doi:10.1089/dia.2019.0148
- Buckingham B, Sherr J, Prestrelski SJ, Conoscenti V. Pharmacodynamics, pharmacokinetics, safety, and tolerability of a ready-to-use, room temperature, liquid stable glucagon administered via an autoinjector pen to youth with type 1 diabetes. Pediatr Diabetes. 2022;23(6):754-762. doi:10.1111/pedi.13360
- Gvoke HypoPen [instructions for use]. Chicago, IL: Xeris Pharmaceuticals, Inc.
INDICATION AND IMPORTANT SAFETY INFORMATION
GVOKE is indicated for the treatment of severe hypoglycemia in adult and pediatric patients with diabetes ages 2 years and above.
IMPORTANT SAFETY INFORMATION
Contraindications
GVOKE is contraindicated in patients with pheochromocytoma because of the risk of substantial increase in blood pressure, insulinoma because of the risk of hypoglycemia, and known hypersensitivity to glucagon or to any of the excipients in GVOKE. Allergic reactions have been reported with glucagon and include anaphylactic shock with breathing difficulties and hypotension.
Warnings and Precautions
GVOKE is contraindicated in patients with pheochromocytoma because glucagon may stimulate the release of catecholamines from the tumor. If the patient develops a dramatic increase in blood pressure and a previously undiagnosed pheochromocytoma is suspected, 5 to 10 mg of phentolamine mesylate, administered intravenously, has been shown to be effective in lowering blood pressure.
In patients with insulinoma, administration of glucagon may produce an initial increase in blood glucose; however, GVOKE administration may directly or indirectly (through an initial rise in blood glucose) stimulate exaggerated insulin release from an insulinoma and cause hypoglycemia. GVOKE is contraindicated in patients with insulinoma. If a patient develops symptoms of hypoglycemia after a dose of GVOKE, give glucose orally or intravenously.
Allergic reactions have been reported with glucagon. These include generalized rash, and in some cases, anaphylactic shock with breathing difficulties and hypotension. GVOKE is contraindicated in patients with a prior hypersensitivity reaction.
GVOKE is effective in treating hypoglycemia only if sufficient hepatic glycogen is present. Patients in states of starvation, with adrenal insufficiency or chronic hypoglycemia, may not have adequate levels of hepatic glycogen for GVOKE administration to be effective. Patients with these conditions should be treated with glucose.
Necrolytic migratory erythema (NME), a skin rash commonly associated with glucagonomas (glucagon-producing tumors) and characterized by scaly, pruritic erythematous plaques, bullae, and erosions, has been reported postmarketing following continuous glucagon infusion. NME lesions may affect the face, groin, perineum and legs or be more widespread. In the reported cases NME resolved with discontinuation of the glucagon, and treatment with corticosteroids was not effective. Should NME occur, consider whether the benefits of continuous glucagon infusion outweigh the risks.
Adverse Reactions
Most common (≥5%) adverse reactions associated with GVOKE are nausea, vomiting, injection site edema (raised 1 mm or greater), and hypoglycemia.
Drug Interactions
Patients taking beta-blockers may have a transient increase in pulse and blood pressure when given GVOKE. In patients taking indomethacin, GVOKE may lose its ability to raise blood glucose or may even produce hypoglycemia. GVOKE may increase the anticoagulant effect of warfarin.
Please see the Full Prescribing Information for Gvoke.